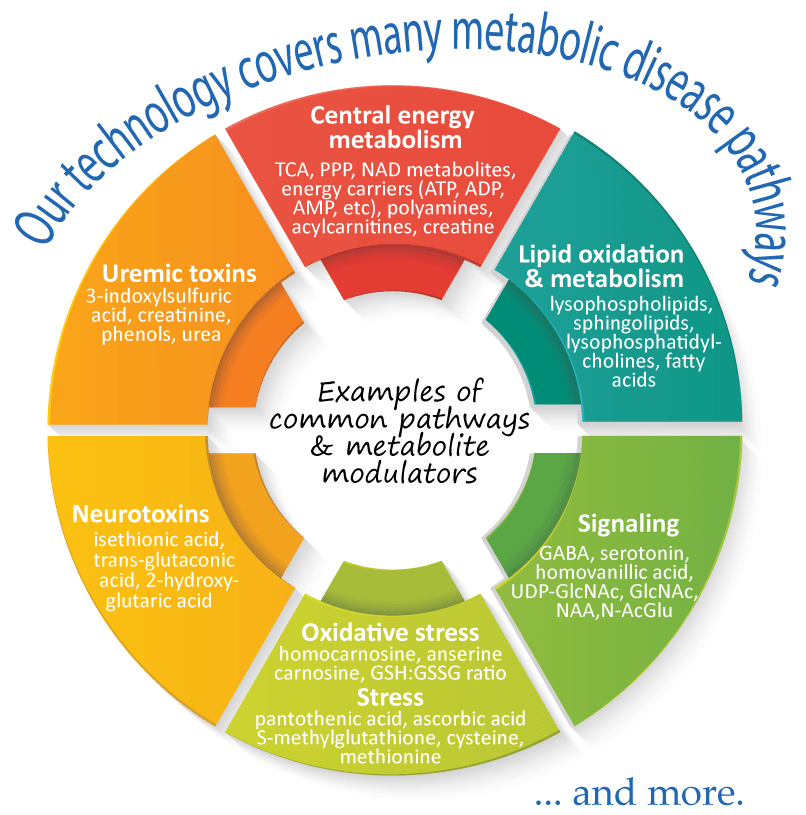
Metabolomics comprehensively characterizes small polar and lipid metabolites, yielding a snapshot of physiological processes that vary according to the pathological state of cells, tissues, and organs. Therefore, metabolomics profiling can help to reveal specific metabolic changes, for example, aberrant levels of amines, amino acids, and lipids, that are known to associate with many metabolic diseases, such as obesity, diabetes, and cardiovascular diseases. Furthermore, metabolomics has a great potential for identifying biomarkers, as well as predictive and risk markers that are associated with disease development and progression, thus, providing an effective strategy for novel therapeutic innovations.
Metabolomics brings us closer to the phenotype of an individual, providing a direct readout of metabolite alterations that occur in many metabolic diseases.